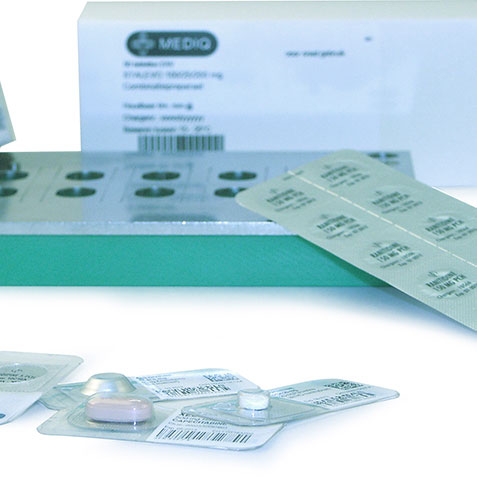
The Unit Dose Pack solution.
What makes UDP unique?
Method 1 – The traditional EAV, a Dutch acronym for Single Unit Dispensing Packages. This method involves removing the medicine from its original packaging, repacking it and providing it with a barcode and necessary information.
Method 2 – The UDP method, with this method retains the original primary packaging of the medicine, and UDP uses the blister as the exit packaging. UDP provides secondary packaging that has standardised dimensions and has been thoroughly tested by the manufacturer for ergonomic usability for nursing. The secondary packaging contains essential, legible information that identifies the medicine plus a barcode that can include the GTIN number, expiry date and lot number.
UDP B.V. developed and patented this method in order to provide the EAV product with barcode faster and more efficiently without limitations in terms of:
Dimensions
Toxicity
Hygroscopic properties
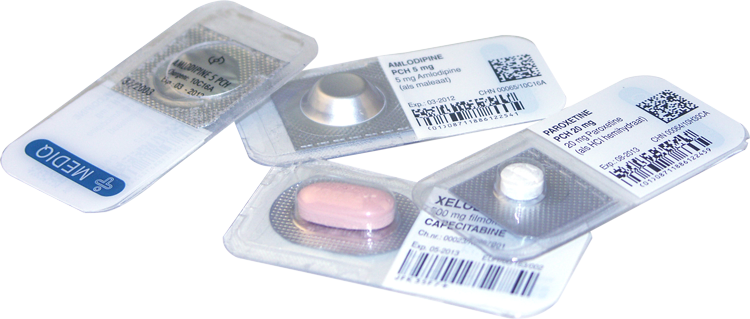
The differences between traditional methods and the UDP method:
The biggest drawback to the EAV method is that the medicines must be removed from their original packaging, meaning that:
· Manufacturing warranties may be lost.
· Shelf-life must be revalidated.
· Stringent requirements apply to production areas and quality systems (moisture, air quality, toxicity of active substances).
· Risks exist for operators, particularly as regards the toxicity of active substances, and protective clothing is required.
· Expensive packaging must be destroyed.
· Every switch to a different medicine necessitates removing residual substances from all parts.
· These circumstances tend to make production very expensive, particularly for small production runs.
The traditional repacking methods interrupts the tracking and tracing chain from manufacturer to patient. Counterfeit medicines are one of the biggest enemies of the pharmaceutical industry. UDP is leaving medicines in their original packaging, which can be provided with algorithms in combination with invisible ink (Anti-Counterfeiting Feature), means the medicine can be traced until after being taken by the patient.
For the UDP® product a worldwide patent was applied and registered at the Netherlands Patents Centre on May 4, 2007.